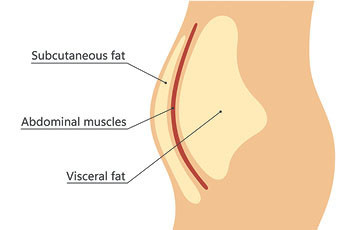
Everybody knows that excessive fat can lead to a wide variety of health issues. Obesity is known to increase the risk of cardiovascular disease, type 2 diabetes, hypertension, and sleep disorders. But not all fat is created equal in terms of health risks. It turns out that visceral fat, which surrounds the organs inside the abdominal wall and is differentiated from the subcutaneous fat found just beneath the skin, is directly linked to poor cardiovascular health. Excess visceral fat, independent of other conditions, leads to cardiovascular diseases including heart failure, atherosclerosis, sudden cardiac death, and atrial fibrillation.
Source: Harvard Medical School
Readers are encouraged to scan the article linked above, which is an extensive scientific statement from the American Heart Association on the current (as of 2021) evidence surrounding the link between excess fat, especially visceral adipose tissue (VAT), and a range of cardiovascular diseases. About halfway through the article there is a section specifically focused on heart failure (HF) and arrhythmias. There is a very detailed discussion of the mechanisms by which VAT leads to heart failure.
The scientific evidence is pointing to the need for people to reduce VAT in the abdominal cavity and surrounding the heart and lungs. Increased exercise (especially aerobic exercise) and decreased caloric intake have demonstrated mostly positive but mixed results in studies of their ability to specifically reduce VAT. The exercise and diet routine is notoriously difficult for people to maintain, and for many heart patients adequate exercise may not even be feasible.
New Research Points to Potential VAT Reduction
Cardiol Therapeutics Inc. (Nasdaq: CRDL) (TSX: CRDL) recently announced compelling study results, presented at the Heart Failure Society of America Annual Scientific Meeting 2023, of the effectiveness of subcutaneously administered cannabidiol for the treatment of heart failure with preserved ejection fraction (HFpEF). The active pharmaceutical ingredient (API) in Cardiol’s novel CRD-38 subcutaneous formulation, cannabidiol, demonstrated multiple cardioprotective effects when used in a model that mimics the conditions found prominently in HFpEF patients. The API prevented undesirable increases in crucial cardiac inflammatory and remodeling markers.
Perhaps most importantly, subcutaneously administered cannabidiol slowed increases in both body and heart weight and reduced the ratio of VAT to SAT (subcutaneous adipose tissue). An imbalance in this ratio with excessive VAT is associated with a higher risk of cardiovascular disease, including HF. VAT accumulation increases cardiac strain, promotes high blood pressure, and dysregulates lipid metabolism. Additionally, it can lead to obesity-related comorbidities such as diabetes. This type of direct action, should it be proven in further studies, could be a game changer for the treatment of all VAT-related cardiovascular conditions.
Cardiol is focusing its study on heart failure, which is a leading cause of death and hospital admissions both domestically and globally. It is estimated heart failure generates about $30 billion of annual healthcare spending in the United States alone. HFpEF is increasing in prevalence and now accounts for about 50% of cases of heart failure. The prognosis for HFpEF patients is grim, with a 5-year mortality rate exceeding 50%.
Based on this encouraging pre-clinical research, Cardiol is now gathering evidence to support an Investigational New Drug application (IND) with the Food and Drug Administration (FDA). Subject to the completion of additional studies, Cardiol anticipates filing an IND with the FDA to commence testing in humans next year.
Cardiol’s Phase II Clinical Programs in Acute Myocarditis and Recurrent Pericarditis
In addition to the CRD-38 program, Cardiol is conducting two simultaneous Phase II studies for the treatment of rare heart diseases: recurrent pericarditis and acute myocarditis. Pericarditis is inflammation of the pericardium, the sac surrounding the heart. Myocarditis is inflammation of the myocardium, the heart muscle itself. Myocarditis has been getting some press lately as it is a rare but known side effect of COVID-19 infection.
Both conditions are rare but debilitating for those suffering from the diseases and the cost of treatments can be prohibitively expensive. Pericarditis is estimated to affect about 160,000 Americans annually, with about 18,000 of those cases requiring hospitalization. Almost a third of pericarditis patients will experience a recurrence, and the most persistent cases can last for years. The only FDA-approved therapy for recurrent pericarditis costs over $150,000/year.
There are no FDA-approved therapies for acute myocarditis, which remains a leading cause of sudden cardiac death in people less than 35 years of age. People suffering from acute myocarditis, which is estimated to affect approximately 46,000 Americans each year, can be hospitalized for prolonged periods and even require intensive care while suffering from complications including cardiac arrest and heart failure.
Cardiol is investigating an oral formulation of cannabidiol, CardiolRx™, in its two Phase II studies for these conditions. The company intends to pursue orphan drug status with both the FDA and the European Medicines Agency, pending the outcomes of its studies. The orphan drug programs are designed to encourage development of treatments for rare diseases that might otherwise fly under the radar of pharmaceutical researchers and can provide CardiolRx™ with seven to ten years of market exclusivity in billion-dollar markets.
The Upshot
Cardiol Therapeutics, as the name suggests, is committed to developing therapies for a variety of life-threatening heart conditions. Its management team, Board of Directors, Scientific Advisory Board, group of clinical trial advisors consists of cardiac health veterans and luminaries that represent a cross-section of the finest heart scientists in the world. Years of research has led the company to this point, developing novel therapeutics for some of the most vexing heart conditions that have so far eluded adequate solutions.
Cardiol’s recent research breakthrough shows the potential for CRD-38 to directly reduce the ratio of VAT to SAT and prevent increases in cardiac inflammatory and remodeling markers, pointing the way to a potential treatment for heart failure, one of the biggest and most deadly heart conditions. If early evidence is confirmed in additional studies, which are currently underway, the VAT to SAT effect could have broader implications for conditions beyond heart failure. Combining this potential with the actively enrolling Phase II clinical studies for acute myocarditis and recurrent pericarditis, the emerging picture is that of a potential powerhouse in the pharmaceutical cardiovascular industry. There is much more to come from Cardiol Therapeutics and investors are encouraged to take notice.